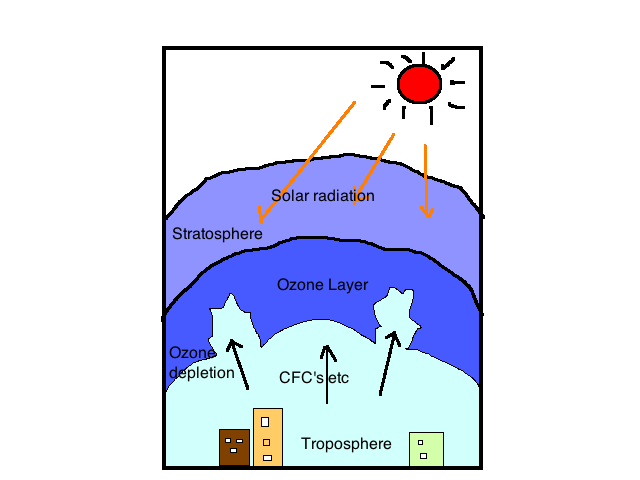
It is a thick layer present in the atmosphere which prevents the harmful rays of the sun mainly Ultraviolet rays to reach the ground. This layer has very high concentrations of Ozone molecules. Its molecular formula is O3. The ozone layer extends up to the area of some parts of the stratosphere and troposphere approximately 15-35 kilometres. This was unknown before the year 1913.
SEPTEMBER 16 is celebrated as INTERNATIONAL DAY for the Preservation of OZONE LAYER
:- Announced by UNITED NATION GENERAL ASSEMBLY
Discovery
The ozone layer was discovered by the French scientist CHARLES FABRY and HENRI BUISSON in the year 1913. The most interesting fact observed by these scientists was that he found the ultraviolet rays having a frequency of more than 310 nm while measuring the frequency that was coming to the Earth from the sun. This mystery of missing all ultraviolet rays of frequency less than 310 nm, led the scientists in deep thought. Finally, scientists came to know about the OZONE layer. A British meteorologist G.M.B. Dobson had explored the ozone in very detail. To honour him, the unit used to measure concentrations of ozone is kept as The DOBSON UNIT". He had made his own device called "spectrophotometer" [commonly known as Dobson meter] which he used to observe the conditions of the ozone layer. The ozone layer was discovered by the French scientist CHARLES FABRY and HENRI BUISSON in the year 1913. The most interesting fact observed by these scientists was that he found the ultraviolet rays having a frequency of more than 310 nm while measuring the frequency that was coming to the Earth from the sun. This mystery of missing all ultraviolet rays of frequency less than 310 nm, led the scientists in deep thought. Finally, scientists came to know about the OZONE layer. A British meteorologist G.M.B. Dobson had explored the ozone in very detail. To honour him, a unit used to measure the concentrations of ozone is kept as The "DOBSON UNIT". He had made his own device called "spectrophotometer" [commonly known as Dobson meter] which he used to observe the conditions of the ozone layer.
Formation of OZONE
The ozone layer is mainly a result of photochemical mechanisms. This was found by the scientist in the year 1930 by SYDNEY CHAPMAN. And, so the formation of ozone is just by breaking down the chemical bond of Oxygen (O2) into oxygen atom. This is known as Photo disassociation. After that combining the oxygen atom with the molecular oxygen. It is assumed that the formation of this ozone layer have might take 2 billion years. And the presence of the ozone layer is the main reason for the existence of life on Earth. However, the molecule of ozone is not stable, so whenever Ultraviolet light strikes with the ozone, the ozone gets splits into an oxygen atom and molecule of oxygen. It can be stated as :
1]. O3 + Ultraviolet rays ------- Oxygen molecule (O2) and oxygen atom 2]. O2 + Oxygen atom ------- Ozone
This is a continuous chain and it is known as Oxygen cycle.
OZONE DEPLETION:-
The ozone layer absorbs up to 97% - 99% UV rays arriving from the sun towards Earth. As we have discussed above. during absorption of the UV rays, the ozone molecule decomposes to an Oxygen molecule and an individual oxygen atom. But over several decades, human activities also led to damage ozone. By the year 1970s ozone layer depletion were revealed by various research. There are many factors, which are affecting the ozone layers, but the most important gas is CFC's "chlorofluorocarbon", bromofluorocarbons and halocarbon. CFC's is mainly used in refrigerators to keep the stored item cold, and in propellent devices and another Nitrous oxide, nitric oxide, hydroxyl, and many more These are the very harmful gases that break and form new compounds with oxygen.
Saved
This led to the depletion of ozone and a gateway to enter harmful ultraviolet rays. It is assumed that the radical of these gases can break the ozone by having 100,000 molecules. Nitrous oxide is said to be the largest Ozone Depleting Substance (ODS) produced/emitted by human activities by the year 2009.
OZONE HOLE
It refers to the depletion of layers of ozone mainly of the stratosphere caused due to the emission of unwanted harmful chemicals as well as ultraviolet rays. As the word say it as a hole, but it is not a hole. Initially, it was observed in the Antarctic region.
We get this such type of information just because of the satellite. The purple and blue colour of the diagram indicates the very low amount of presence of Ozone while yellow and red colour represents a good amount of presence of oxygen. As we get to know from the above Discovery subheading, the ozone layer having values less than 220 Dobson Units was not known before 1979. Thus 220 Dobson unit is as represents the boundary of the ozone layer. However, this ozone layer is found only in the ANTARCTIC area.
Why the OZONE hole found only in Antarctica?
As we know that Ozone-depleting substances such as CFC's, Nitrogen oxide, Sulphur dioxide are spread all over the world in the stratospheric region. But the question arises, that why the ozone hole is present only in the Antarctica region. This just because of the special type of atmospheric conditions and temperature of the Antarctica region that is found only here. Since it is located at the Pole, so the temperature is very low. Firstly you should know that some chemicals which have a large amount of atmospheric chlorine-like HCL short of Hydrochloric acid do remain stable in the normal atmospheric conditions or temperature. But, the temperature on the pole side is different from normal conditions. During winter polar vortex isolates the air in the centre. It led to the formation of Polar Stratospheric Clouds {PSC's}. The special reaction that occurs with PSC's can only happen on the surface of the Polar Stratospheric clouds particles; doesn't matter in which form they are. This reaction helps in the activation of Chlorine Cl2 gas. Thus when sunlight again rises in Antarctica after 6 months, the ultraviolet rays easily break the bond between the chlorine atoms and these free chlorine atoms participate in the destruction of the Ozone layer.
How OZONE Layer can be replenished?
Ozone is a self-replenishing gas. But, the time required for its replenishment is in our hand. We can increase or decrease the speed of replenishment. Let's understand this by an example: If we will use ODS like CFC's, Sulphur dioxide then it will surely damage the Ozone layer so, during replenishment, these damages will decrease it momentum while it would be faster.